961 | Add to Reading ListSource URL: www.scot-ship.ac.ukLanguage: English - Date: 2014-04-01 08:14:48
|
|---|
962 | Add to Reading ListSource URL: presentations.copernicus.orgLanguage: English - Date: 2015-05-07 06:42:18
|
|---|
963 | Add to Reading ListSource URL: www.hma.euLanguage: English - Date: 2016-05-16 05:10:10
|
|---|
964 | Add to Reading ListSource URL: www.fercap-sidcer.orgLanguage: English - Date: 2012-02-08 01:31:08
|
|---|
965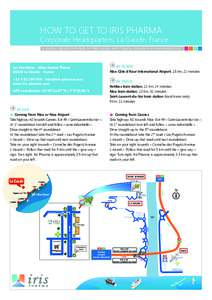 | Add to Reading ListSource URL: www.iris-pharma.comLanguage: English - Date: 2014-05-23 12:41:20
|
|---|
966 | Add to Reading ListSource URL: research.missouri.eduLanguage: English - Date: 2015-07-01 12:43:02
|
|---|
967 | Add to Reading ListSource URL: www.ekon.sun.ac.zaLanguage: English - Date: 2012-06-04 10:01:36
|
|---|
968 | Add to Reading ListSource URL: www.dzne.deLanguage: English - Date: 2016-06-13 10:04:50
|
|---|
969 | Add to Reading ListSource URL: www.fercap-sidcer.orgLanguage: English - Date: 2012-02-08 01:29:56
|
|---|
970 | Add to Reading ListSource URL: ccnmtl.columbia.eduLanguage: English - Date: 2007-10-05 14:09:59
|
|---|